Aptamers & Antibodies: A Cooperation, Not a Competition
Aptamers & Antibodies: A Cooperation, Not a Competition
Reviewing the utility of both aptamer and antibody reagents in your discovery applications
Introduction
Since their first reported use in immunoassays, oligonucleotides have made significant advancements for their application in detection assays. Most notably, as aptamers in a sandwich assay called ELONA. Aptamers have since been coined the direct alternative to antibodies as either capture or detection reagents in discovery platforms.
We cover the technical specifics of ELONA assays in a separate technical note. Find it here
With several studies presenting competitive analysis of the two reagents and some going as far as suggesting the potential of aptamers to completely replace antibodies-we feel that it is necessary to depress some of the exaggerations promoting this rivalry. In principle, both options offer their own features and aptamers do seem to have advantages in some key areas such as size, multiplexing capabilities, and cost. However, as is everything in science; things are not always as straightforward as they seem and this topic is no exception.
In this piece, we review the conventional aptamer : antibody side by side comparison, use our professional experiences with both reagents to highlight some essential oversights between the two, and make a case for implementing both reagents in your experimental workflow to reinforce your discovery platform.
Side-by-Side Comparison
The traditional case between aptamers and antibodies primarily focus on two factors; Biophysical structure and target affinity/specificity.
Physical properties
In figure 1 we show a common image for the size representation of antibodies relative to aptamers. The significantly lower molecular weight of aptamers suggests a series of advantages including; versatility, low toxicity, and cheaper cost manufacturing benefits. Although, these may be safe theories on paper-the specifics behind the material science in manufacturing these reagents is more complicated. For example, aptamers often become highly modified oligonucleotides as they develop to their target which disrupts their stability. In order to counter the loss in stability and retain purity, additional chemical modifications are required, such as phosphothioation of the oligomer backbone. All these modifications require more attention and specialty expertise in synthesis for production. In turn, increasing both the cost and toxicity risk of the final product.
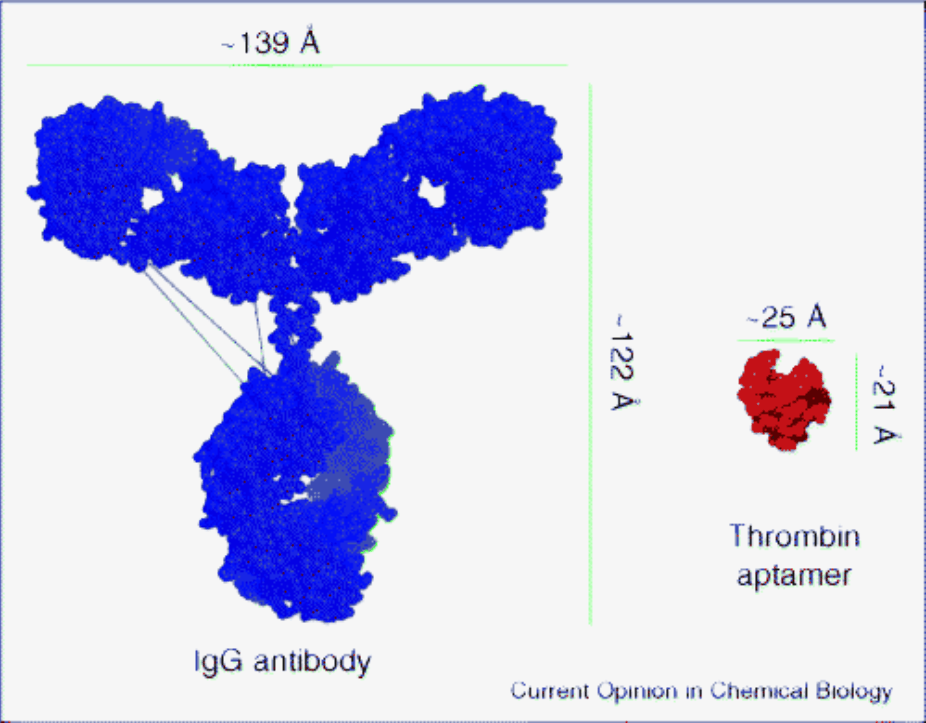
Fig 1. Size: Antibody vs. Aptamer. Opinion in chem. bio
Affinity & Specificity
While affinity to proteins ranges from nanomolar to picomolar, aptamers against small molecules tend to have an affinity in the micromolar range (Kd ~ μM). Both aptamers and antibodies are highly specific to their target molecules. However, aptamers are able to discriminate enantiomers and protein isoforms during detection.
Figure 2 provides a useful side-by-side analysis outlining the observed differences in affinity properties between oligo-aptamers and protein antibodies.
| Aptamers (oligonucleotides) | Antibodies (proteins) |
|---|---|
| Binding affinity is in low nanomolar to picomolar range | Binding affinity is in low nanomolar to picomolar range |
| Selection is an in vitro process that can target any small molecule, biopolymer, or cell | Selection requires a biological organism and is inefficient with toxins and small non-immunogenic molecules |
| Selection of aptamers is inexpensive and takes few weeks | Screening of monoclonal antibodies is expensive and time consuming |
| Uniform activity regardless of the batch | Activity of antibodies varies from batch to batch |
| Affinity parameters can be controlled on demand (“smart aptamers”) | Difficult to modify affinity parameters |
| Wide variety of chemical modifications are introduced to diversify properties and functions | Limited modifications |
| Return to original conformation after temperature insult | Temperature causes irreversible denaturation |
| Unlimited shelf-life | Limited shelf-life |
| No evidence of immunogenicity | Significant immunogenicity |
| CE analysis: non-sticky to capillary walls, light ligands (5-15 kDa), easy to separate Apt•P from Apt | CE analysis: sticky to capillary walls, bulky (150 kDa), difficult to separate Ab•P from Ab |
Fig 2. Aptamers rival antibodies in affinity analysis (Science, 2000, 287, 820-825)
Rivalry Oversights
Based on the current comparative studies; aptamers generally, do offer preferable advantages to a laboratory’s scientific projects and material budget. Nonetheless, it remains that these studies lack consideration of certain factors that are key in providing a complete understanding these reagents -as research tools. Factors such as their commercial landscape and mechanistic chemistry are too challenging for traditional studies to communicate alongside their analytical design. To help clarify on this point, we translated our diverse commercial experiences with these reagents into examples of some of the key factors being disregarded respectively:
Aptamer patents
Contrary to monoclonal technology, the initial patents surrounding aptamers were heavily controlled. There are notable recent changes, as some of those patents begin to lapse and alternative open methods develop. However, intended downstream application of these reagents for diagnostic or therapeutic use still requires extra due diligence on proper rights early in the discovery research phase.
The mAb standard
Monoclonal antibodies have been an established global standard in a large majority of laboratories for many years. The process of replacing a standard in bioscientific research involves widespread validation over a significant period. So even if there is a likelihood of succession, it would still require long, comprehensive consistency which may cause delays and/or surprises in downstream data criteria for anyone pioneering sole-aptamer platforms.
Biochemical limitations
The counter-selection factor during nucleic acid partitioning of the background library is not always able to preclude unwanted aptamers relative to the immobilizing label’s -which in turn complicates the aptamer design. Ironically, the biophysical characteristics of nucleic acids; (ex. 2' amine and 2'-O-Me) aptamers turns out to be much more restricted than that of antibodies. Since oligonucleotides are very hydrophobic and negatively charged by design-binding to targets on proteins that are hydrophobic or acidic becomes more challenging than the scientists expect.
Conclusion
Despite the pessimistic tone of this piece, I am actually a very strong believer in aptamers. They have great potential in their advantages against antibodies. However, it is important to remain more pragmatic than optimistic when it comes to their current role in laboratory research. Particularly if our goal is to effectively enable their advancement. To do that, aptamers need to stop being looked at as competing reagents for antibodies and instead be considered as partners for application alongside antibody experiments. In other words, antibodies have established themselves in certain methodologies, where the advantage of aptamers pose little to meaningless innovation. It is new and relevant areas of research, where the utility of aptamers shows great potential and offer the greatest return in capitalizing on their advantages.
For example; published studies, using aptamers as specific inhibitors against diverse target protein families in vitro and in vivo-highlight their potential as effective inhibitors and degraders for various drug targets. Focusing on their application in such promising areas where antibodies are not as established helps establish both aptamers and the principal research objective. If this is done alongside established antibody applications throughout a discovery workflow, it would be the most ideal approach for utilizing the advantages of both aptamers and antibodies without unnecessary bias.
References
- Annu. Rev. Med 2005, 56, 555-83.
- Science, 2000, 287, 820-825
- Translational Medic S1:001. doi:10.4172/2161-1025. S1-001
- Banner image by Biorendr















