Bioconjugation Chemistry: Challenges and Solutions
Bioconjugation Biochemistry: Challenges and Solutions
Bioconjugation represents the derivatization of biomolecules (proteins, carbohydrates, nucleic acids) which allows the site-specific creation of a covalent link between a biomolecule and an exogenous moiety in order to endow desirable properties using a different set of techniques.1,2 Bioconjugation biochemistry enables the creation of hybrid molecules that exhibit the properties of both biomolecules and exogenous moieties. Examples of bioconjugation based hybrid molecules include protein structure elucidation using tags, enzyme immobilization, antibodies binding to fluorophores, cellular imaging, microarrays, etc.3,4. Bioconjugation typically involves the modification of biomolecules by adding distinct but complementary functional groups through a wide range of chemical techniques/reactions using different linkers. However, to develop new methodologies, site specific conjugation continues to garner much attention to match the ever-increasing requirements of preserving biomolecule integrity, stability, and mildness. Nevertheless, bioconjugation techniques have emerged as a powerful set of tools with applications in ground-breaking targeted biotherapeutics, disease diagnosis, ligand discovery, high throughput drug screening, biosensors.5,6,7
Criteria for successful bioconjugation:
Any functional bioconjugation strategy should meet certain criteria irrespective of the techniques employed
- The bioconjugation reactions must take place in aqueous solutions in a controlled manner to maintain the biomolecules’ inherent functions.
- The conjugation reaction should be stoichiometrically efficient with fast kinetics.
- There should not be any additional agents which could potentially disrupt the biomolecules’ functions such as an oxidant, reductant, or metal during conjugation reaction.
- There should not be any covalent side reactions during modification or conjugation.
- Conjugate bonds should not form between endogenous functional groups present within the biomolecules, instead conjugate bonds should form only between the two complementary linkers incorporated on the biomolecules during modification.
- Linkers should be easily incorporated on a variety of biomolecules (oligos and peptides) during solid-phase synthesis.
- The quantitation of linker incorporation and conjugate formation should be easy through simple and non-destructive methods (For example, spectrophotometry).
- The resulting bioconjugate should be stable under broad pH and temperature ranges to meet a variety of experimental needs.
Though various methodologies are currently being employed for the bioconjugation of biomolecules, still many methods are facing challenges, thus limiting their general applicability. Moreover, several critically important bioconjugation parameters have been completely neglected in the past, which needs to be addressed properly to increase the overall bioconjugation efficiency. For example, the number of samples utilized, the stability of the bioconjugation linkage, and the distribution of bioconjugation generated products are of utmost importance and should be given great attention. In addition, more exploration of the sensitivity of analytical methodologies is necessary to develop efficient and selective bioconjugation methods. In recent years, the advancement of bioconjugation techniques for the development of vaccines, emerging biomaterials, and new therapeutic conjugates has further necessitated the consideration of biocompatibility, biostability and bioselectivity in the context of the challenges associated with the bioconjugation methods being utilized. In this context, we have highlighted below the most important types of bioconjugates (protein bioconjugates, antibody bioconjugates, antibody-enzyme bioconjugates, protein-DNA bioconjugates), their associated challenges, and possible solutions to those challenges.
Protein Bioconjugates:
Proteins are the most utilized biomolecules in bioconjugation biochemistry, and are generally leveraged for labelling live proteins, stabilizing proteins being used in protein-based therapies, cellular imaging, and biological processes monitoring. The selection of a protein bioconjugation methodology primarily depends on:
- The intrinsic reactivity of the targeted amino acid residue in the protein (oxido-reductive characteristics, acidity/basicity, electrophilicity/nucleophilicity),
- Its protein-specific environment related to accessibility, whether its N- or C- terminal ends, the location of amino acids within the specific sequence or chain, etc...
Proteins greatly vary in terms of their modification as some proteins are easy to manipulate while others face problems during bioconjugation. Protein modification methods also widely vary related to the protein’s properties, such as functional group compatibility, inherent site selectivity, and overall yield7,8. In recent years, various biochemical techniques and approaches have been utilized for the bioconjugation of proteins for the development of new protein-drug conjugates, targeted medical imaging agents, protein-hybrid materials with complex functions, and the improved understanding of biological processes.
Challenges with Protein Bioconjugates:
The challenges associated with protein bioconjugates include a lack of site-specificity, lack of access to the reaction site, and a lack of bioconjugation techniques for different amino acids.
Lack of Site Specificity
In some protein bioconjugation methods, a lack of site-specificity imposes an inability to create specific reaction sites to target the desired protein during a bioconjugation reaction acids.
Troubleshooting Tips
- Use a catalyst to promote a site-specific protein conjugation reaction.
- Incorporate an unnatural amino acid and synthesize your target protein with it. For example, the site-specific incorporation of an unnatural amino acid at the N-terminal end of a protein.
Lack of Access to the Reactive Site
While dealing with native proteins, the desired reactive groups can be limited or inaccessible. This represents a difficult problem due to protein folding. This protein reactive site inaccessibility leads to poor yields of protein bioconjugates, and sometimes even the failure of the bioconjugation reaction.
Troubleshooting Tips
- Find an alternative reactive site
- Directly modify the protein to attain better access to your target reactive site
- Modify the target protein with genetic manipulation to make it more accessible.
Lack of Bioconjugation Techniques for Different Amino Acids
Different modification methods are available for different amino acids in proteins. Some amino acids are not easy to modify using routine bioconjugation techniques. For example: tyrosine has recently emerged as a bioconjugation target and does not have many bioconjugation techniques.
Troubleshooting Tips
- Modify previously utilized bioconjugation techniques.
- Develop new methods for the bioconjugation of specific amino acids.
Antibody Bioconjugates:
Antibody bioconjugates have also been significantly utilized in the field of bioconjugation science with fascinating applications in biological imaging and immunohistochemistry.
Challenges with Antibody Bioconjugates:
Antibody bioconjugates often encounter problems due to their fragile and complicated nature. The challenges include instability of the bioconjugate product, and degradation of the antibody.
Instability of the Antibody Bioconjugate Product
Once antibody bioconjugates are generated, you may face the issue related to their stability as they have a tendency to decompose immediately or during storage like normal antibodies.
Troubleshooting Tips
- Cool your antibody bioconjugate. Cooling increases the storage life of bioconjugates.
- Avoid freezing the bioconjugates
- Use a specific antifreeze stabilizer in storage solutions to extend the shelf life. Such as 50% glycerol or ethylene glycol to increase the shelf life at -20 degrees.
Degradation of the Antibody
The utilization of harsh chemicals to produce stable chemical bonds between an antibody and another molecule generally leads to the degradation of generated bioconjugates.
Troubleshooting Tips
- Control the reaction conditions. For example, performing the experiment in cooler conditions or in a nitrogen environment reduces degradation.
- Reduce the use of harsh chemicals during bioconjugation. This may be achieved by using milder chemicals or simply changing the solvents or solutions.
Antibody-Enzyme Bioconjugates:
Antibody-enzyme conjugates are promising products for cancer therapy and in the area of immunohistochemistry research.
Challenges with Antibody-Enzyme Bioconjugates:
The challenges with antibody-enzyme conjugates include the degradation of enzyme activity and controlling linker length.
Degradation of Enzyme Activity
The enzyme activity may be negatively affected by the reagents and conditions used in bioconjugation reactions.
Troubleshooting Tips
- Use an enzyme stabilizer to minimize the degradation of bioconjugates
- Use alternative reagents and/or linkers.
- Change the reaction conditions related to pH and solvent
- Use a different conjugation strategy.
Controlling Linker Length
Linker length can range from “zero length” to extended length chains and affect the stability and reactivity of antibody-enzyme conjugates. Therefore, controlling linker length is important to maintain the stability and activity of enzymes in antibody-enzyme bioconjugates.
Troubleshooting Tips
- Experiment with different linker lengths to assess how this affects the performance of the bioconjugates.
- Use alternative linkers with prolonged stability.
- Avoid using linkers that can be easily broken in reaction conditions.
- Avoid peptide-based linkers.
Protein-DNA Bioconjugates:
DNA and proteins represent two of the most important classes of biomacromolecules in biology. Combining DNA and proteins opens several avenues for bioconjugation. Protein-DNA bioconjugates provide tools for structural hybridization, enzymatic catalysis, and molecular recognition.
Challenges with Protein-DNA Bioconjugates:
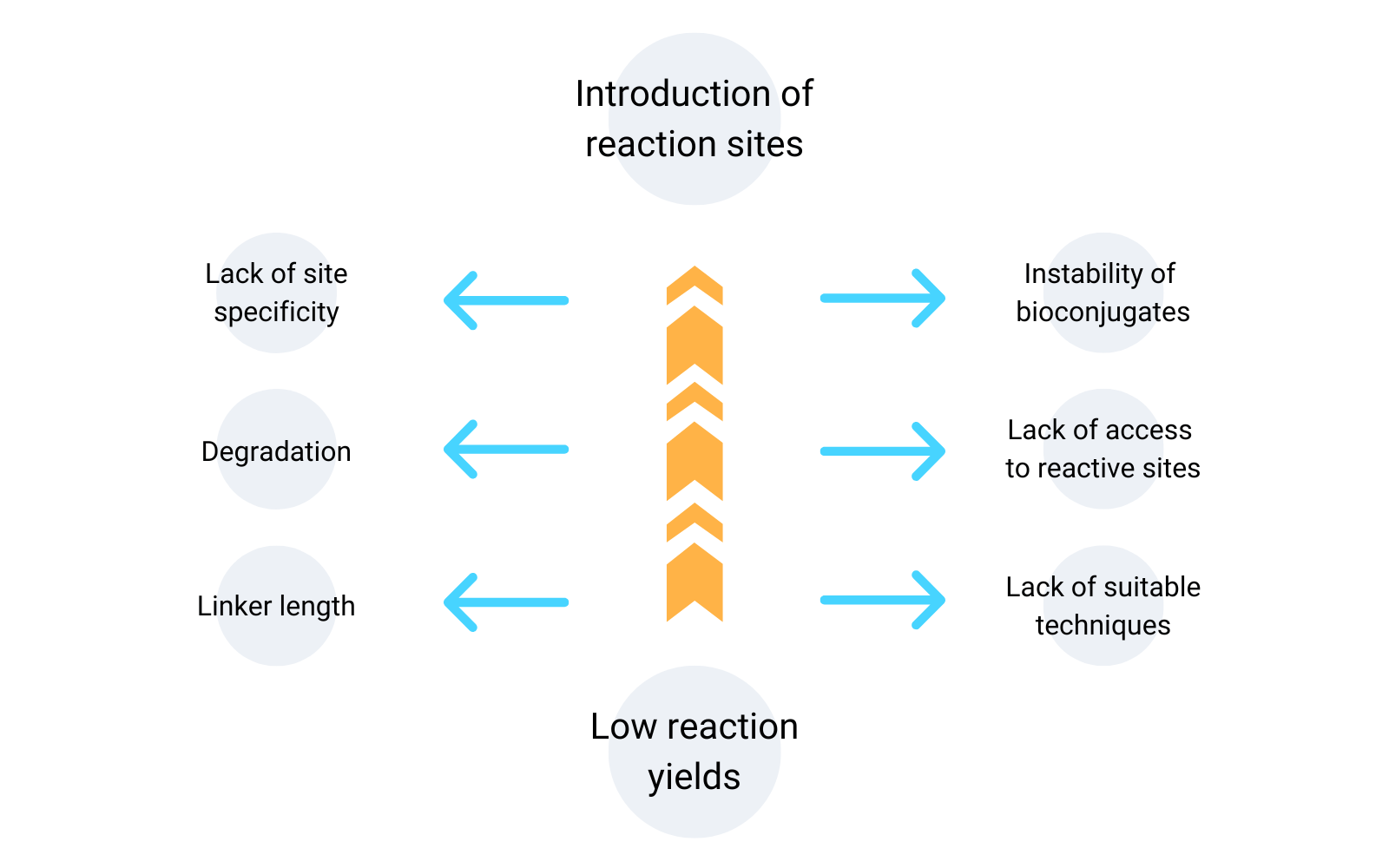
The challenges associated with protein-DNA bioconjugation techniques include low reaction yields and the introduction of reaction sites to the DNA.
Low Reaction Yields
One of the most frustrating problems encountered during the synthesis of protein-DNA bioconjugates is the production of small amounts of your desired bioconjugates.
Troubleshooting Tips
- Optimize your reaction conditions. Using simple tweaks such as tailoring reaction time, solvent, and temperature may improve the yield of bioconjugates.
- Try an alternative purification technique.
Introduction of Reaction Sites On DNA
During protein-DNA bioconjugation, it is necessary to introduce reaction sites to the DNA structure. The introduction of reaction sites on DNA is not straight forward and poses challenges.
Troubleshooting Tips
- Use direct chemical modification to add the desired functional groups to the DNA structure.
- Synthesize DNA with your desired functionality using emerging techniques and approaches. These include techniques such as enzymatic gene synthesis, or microarray mediated gene synthesis.9
References:
- Koniev O, Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem Soc Rev. 2015 Aug 7;44(15):5495-551. doi: 10.1039/c5cs00048c.
- Wadhawan A, Chatterjee M, Singh G. Present Scenario of Bioconjugates in Cancer Therapy: A Review. Int J Mol Sci. 2019;20(21):5243. Published 2019 Oct 23. doi:10.3390/ijms20215243
- Wang RE, et al. 2015. An immunosuppressive antibody–drug conjugate. Journal of the American Chemical Society
- Zhao P, et al. 2018. Highly multiplexed single‐cell protein profiling with large‐scale convertible DNA‐antibody barcoded arrays. Advanced Science
- Kalia J, Raines RT. Advances in Bioconjugation. Curr Org Chem. 2010 January; 14(2): 138–147.
- McMahon NP, et al. 2020. Oligonucleotide conjugated antibodies permit highly multiplexed immunofluorescence for future use in clinical histopathology. Journal of Biomedical Optics
- Chen C, Ng DYW, Weil T, Polymer bioconjugates: Modern design concepts toward precision hybrid materials, Progress in Polymer Science,105, 2020,101241.
- Stephanopoulos N, Francis MB. Choosing an effective protein bioconjugation strategy. Nat Chem Biol. 2011 Nov 15;7(12):876-84. doi: 10.1038/nchembio.720.
- Eisenstein, M. Enzymatic DNA synthesis enters new phase. Nat Biotechnol 38, 1113–1115 (2020). https://doi.org/10.1038/s41587-020-0695-9















